How to Convert Grams to Moles: Top Simple Steps
 |
| Illustrated picture. Photo: Mixi's |
Moles are a standard unit of measurement in chemistry that takes into account the different elements in a chemical compound. Often, amounts of compounds are given in grams and need to be converted to moles.
Grams and Moles of a Chemical Substance
Some of you might own a small scale in your kitchen, which you may have used to measure the amount of flour, sugar, and even meat. When you use your kitchen scale, you may get the amount of the substance in grams, ounces, or pounds. In the same way as in your kitchen, you need to measure the amount of substances in a chemistry laboratory. Chemists use a device called an analytical balance, which measures the amount of a chemical substance or compound, usually in grams.
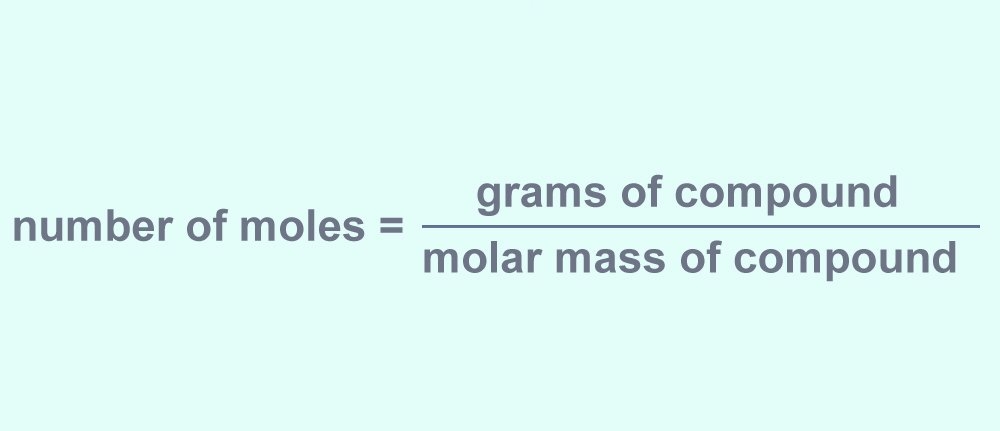
Moles are the standard unit that is used in chemistry to express the amount of a chemical compound. Unfortunately, there is no method to measure moles directly, because we can only physically measure the amount of a compound in grams when we use an analytical balance.
The good thing is, it is possible for us to convert the number of grams of a compound to the number of moles.
Grams to Moles Conversion Problem
 |
| Photo: Science ABC |
Determine the number of moles of CO2 in 454 grams of CO2.
Solution
First, look up the atomic masses for carbon and oxygen from the periodic table. The atomic mass of C is 12.01, and the atomic mass of O is 16.00. The formula mass of CO2 is:
12.01 + 2(16.00) = 44.01
Thus, one mole of CO2 weighs 44.01 grams. This relation provides a conversion factor to go from grams to moles. Using the factor 1 mol/44.01 g:
moles CO2 = 454 g x 1 mol/44.01 g = 10.3 moles
Answer
There are 10.3 moles of CO2 in 454 grams of CO2.
| Example: Convert 25.0 grams of KMnO4 to moles. Solution: 1) Step One: The problem will tell you how many grams are present. Look for the unit of grams. The number immediately preceding it will be how many grams. Common abbreviations for grams include g (just the letter) and gm. I suppose that a problem can be worded in such a way that the number of grams comes after the unit, but that type of trickery isn't very common in high school. The problem gives us 25.0 grams. 2) Step Two: You need to know the molar mass of the substance. Please refer to the lessons about calculating the molecular weight and molar mass of a substance if you are not sure how to calculate a molar mass. The molar mass of KMnO4 is 158.034 grams/mole. Please take a moment and calculate the molar mass of KMnO4, just to be sure. 3) Step Three: Divide the grams given in the problem by the substance's molar mass: 25.0 g ––––––––– = 0.158 mol 158.034 g/mol The answer of 0.158 mole has been rounded to three significant figures because the 25.0 value had the least number of significant figures in the problem. 4) If this problem were set up like the proportion above, you would have this: 25.0 g 158.034 g –––––– = ––––––– x 1 mol 5) Cross-multiply and divide to solve for the unknown. (25.0 g) (1 mol) = (x) (158.034 g) x = 0.158 mol |
Performing Grams and Moles Conversions
Here are some tips for performing these conversions:
- The two problems most commonly encountered are setting up the problem incorrectly, so the units don't cancel out and give the correct result. It helps to write out the conversion and make sure units cancel. You may want to draw a line through them in complex calculations to keep track of active units.
- Watch your significant figures. Chemistry professors are unforgiving when it comes to reporting an answer, even if you set up the problem correctly.
How to Determine Moles in Chemistry
Determining the Molar Mass of an Element
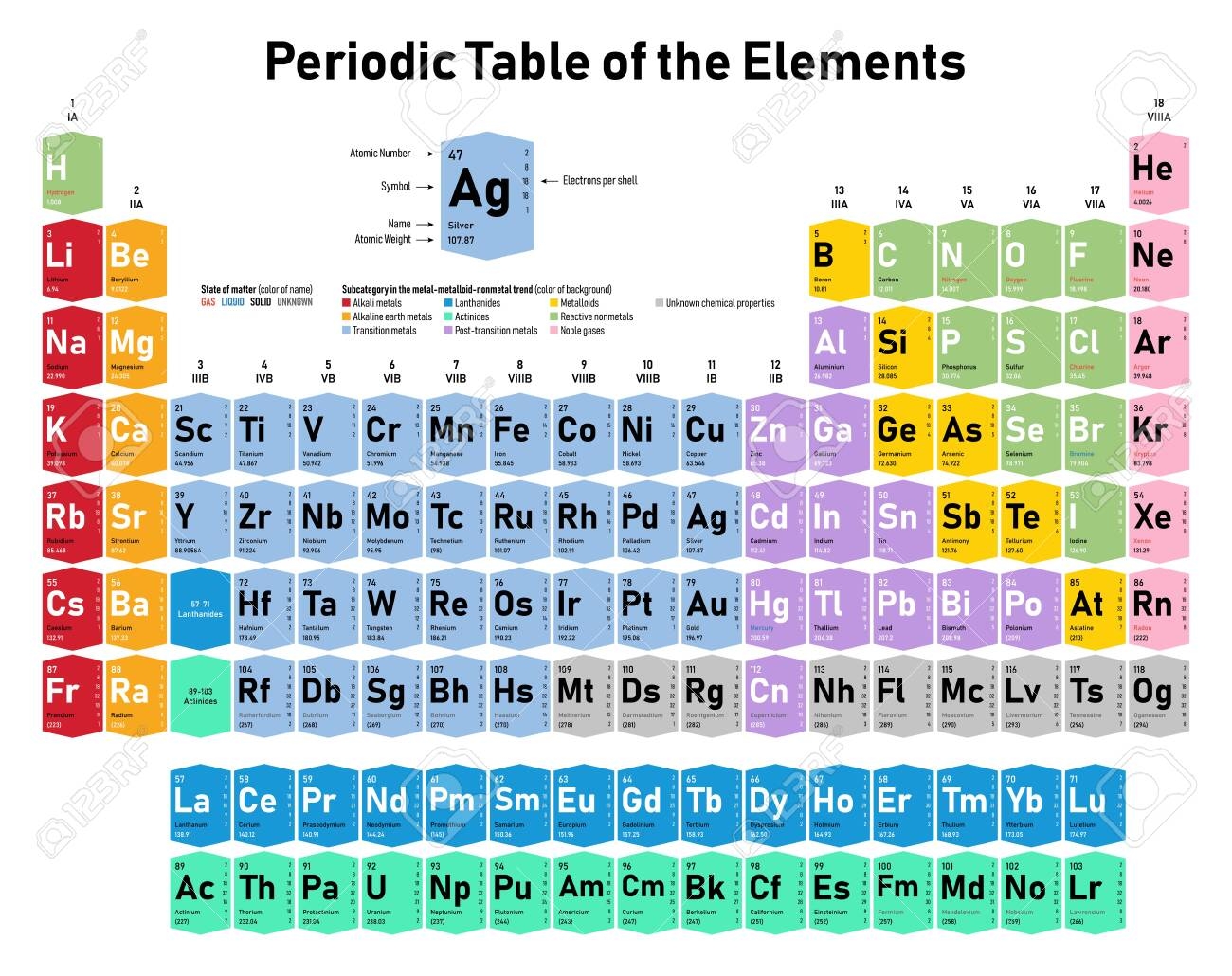
The periodic table of the elements includes each element's atomic number.
Find the element lithium (Li) on the periodic table. The atomic number for lithium is 3, representing the number of protons in the nucleus of one atom.
Note that the mass number of lithium is 6.94, representing the sum of the numbers of protons and neutrons in the nucleus of one atom.
Note that the mass number is equal to the mass (in grams) of one mole of lithium; this is the molar mass of lithium.
Determine the Molecular Mass of a Chemical Compound
 |
| Photo: 123RF |
Determine the molecular mass of carbon dioxide (chemical formula CO2). Find carbon and oxygen on the periodic table.
Note the masses of carbon and oxygen from the periodic table, which are 12.01 and 16, respectively.
Add the mass numbers of one atom of carbon and two atoms of oxygen from the periodic table: 12.01 + 2(16) = 44.01 grams per mole
Converting From Mass to Moles
Calculate the number of moles of water in 600 grams of water (H2O). Find hydrogen and oxygen on the periodic table.
Set up the following equation relating grams to moles:
x moles H2O = (1 mole H2O/18 grams H2O) x (600 grams H2O)
Solve the equation in Step 2 to find that there are 3.33 moles of H2O in 600 grams of H2O.
| Key Takeaways: Converting Moles to Grams (and Vice Versa) * Grams and moles are two units to express the amount of matter in a sample. There is no "conversion formula" between the two units. Instead, you must use atomic mass values and the chemical formula to do the conversion. * To do this, look up atomic masses on the periodic table and use the formula mass to know how many atoms of each element are in a compound. * Remember, subscripts in a formula indicate number of atoms. If there is no subscript, it means there is only one atom of that element in the formula. * Multiply the number of atoms of an element by its atomic mass. Do this for all the atoms and add the values together to get the number of grams per mole. This is your conversion factor. |
In this instruction video, you will learn how to easily convert Grams to Moles:
For more interesting news of KnowInsiders, check out right below!
 How to Convert Minutes to Hours: Easy Ways to Change How to Convert Minutes to Hours: Easy Ways to Change How to convert minutes to hours? Follow our simple steps! |
 How to Convert PDF To Excel: Best Ways to Change How to Convert PDF To Excel: Best Ways to Change Your coworker sent you a PDF file instead of the Excel document you wanted, that doesn’t mean you’re actually stuck with it. In this guide, ... |
 How to Convert Word to PowerPoint How to Convert Word to PowerPoint PowerPoint is a great way to merge text and images for presentations. Are you struggling with the way to convert Word to Powerpoint? Check out ... |