Malaria Vaccine: History, Development & Side effect
 |
| Photo: Science Mag |
What is Malaria?
Malaria is a life-threatening disease. It’s typically transmitted through the bite of an infected Anopheles mosquito. Infected mosquitoes carry the Plasmodium parasite. When this mosquito bites you, the parasite is released into your bloodstream.
The symptoms of malaria typically develop within 10 days to 4 weeks following the infection. In some cases, symptoms may not develop for several months. Some malarial parasites can enter the body but will be dormant for long periods of time. According to Healthline, common symptoms of malaria include: shaking chills that can range from moderate to severe, high fever, profuse sweating, headache, nausea, vomiting, abdominal pain, diarrhea, anemia, muscle pain, convulsions, coma, bloody stools
Malaria is typically found in tropical and subtropical climates where the parasites can live. The World Health Organization (WHO)Trusted Source states that, in 2016, there were an estimated 216 million cases of malaria in 91 countries. In the United States, the Centers for Disease Control and Prevention (CDC) report 1,700 casesTrusted Source of malaria annually. Most cases of malaria develop in people who travel to countries where malaria is more common.
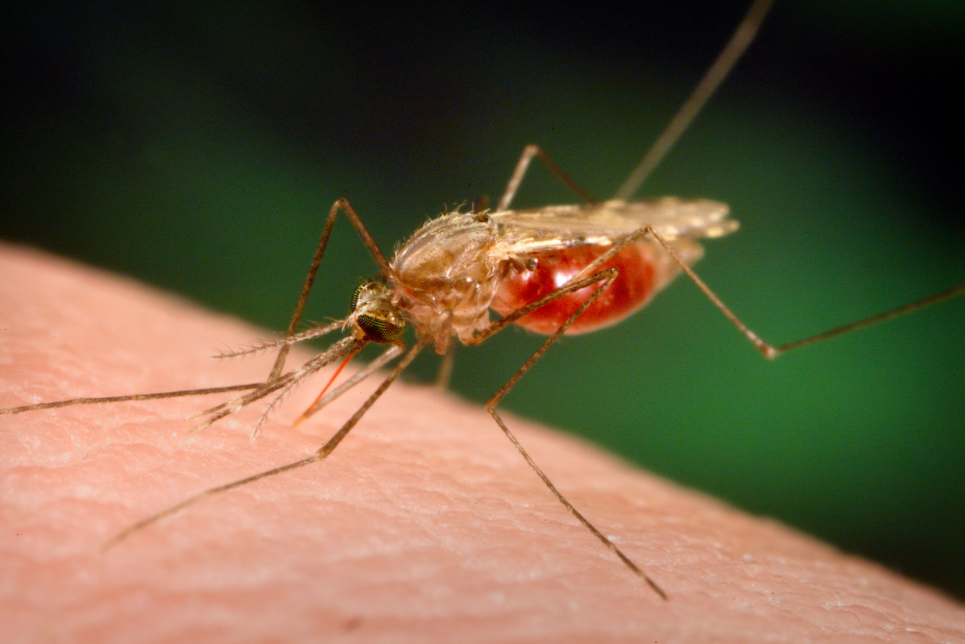 |
| Anopheles mosquito. (Photo: NIH) |
Malaria Vaccine development
Although progress has been made in the last 10 years toward developing malaria vaccines, there is currently no licensed malaria vaccine on the market.
The development of a malaria vaccine has faced several obstacles: the lack of a traditional market, few developers, and the technical complexity of developing any vaccine against a parasite. Malaria parasites have a complex life cycle, and there is a poor understanding of the complex immune response to malaria infection. Malaria parasites are also genetically complex, producing thousands of potential antigens. Unlike the diseases for which we currently have effective vaccines, exposure to malaria parasites does not confer lifelong protection. Acquired immunity only partially protects against future disease, and malaria infection can persist for months without symptoms of the disease.
 |
| Ronald Ross. (Photo: Cosmo) |
Ronald Ross - Nobel Prize winner for the discovery of Anopheles mosquito
The necessity to develop a vaccine against malaria has been emphasized from the identification of the parasite in 1897. In 1897, Ronald Ross discovered the mosquito (vectors) that transmit the disease. Further, only the female Anopheles mosquito can transmit the parasite. However, aiming to develop a highly effective malaria vaccine has led to the use a wide range of new approaches. The emergence of resistant parasites and vectors has caused to concentrate on other controls achievements including a vaccine.
Sir Ronald Ross (1857 – 1932) was a British medical doctor who received the Nobel Prize for Physiology or Medicine in 1902 for his work on the transmission of malaria, becoming the first British Nobel laureate, and the first born outside Europe. His discovery of the malarial parasite in the gastrointestinal tract of a mosquito in 1897 proved that malaria was transmitted by mosquitoes, and laid the foundation for the method of combating the disease. He was a polymath, writing a number of poems, published several novels, and composed songs. He was also an amateur artist and natural mathematician. Ronald Ross was awarded a Nobel Prize for his discovery of the life cycle of malarial parasites in birds. He did not build his concept of malarial transmission in humans, but in birds. Ross made his first important step in May 1895 when he observed the early stages of malarial parasite inside a mosquito stomach. On 21 August, he confirmed the growth of the parasite in the mosquito. This discovery was published on 27 August 1897, in the Indian Medical Gazette and subsequently in the December 1897 issue of British Medical Journal.
 |
| Photo: The Globe Post |
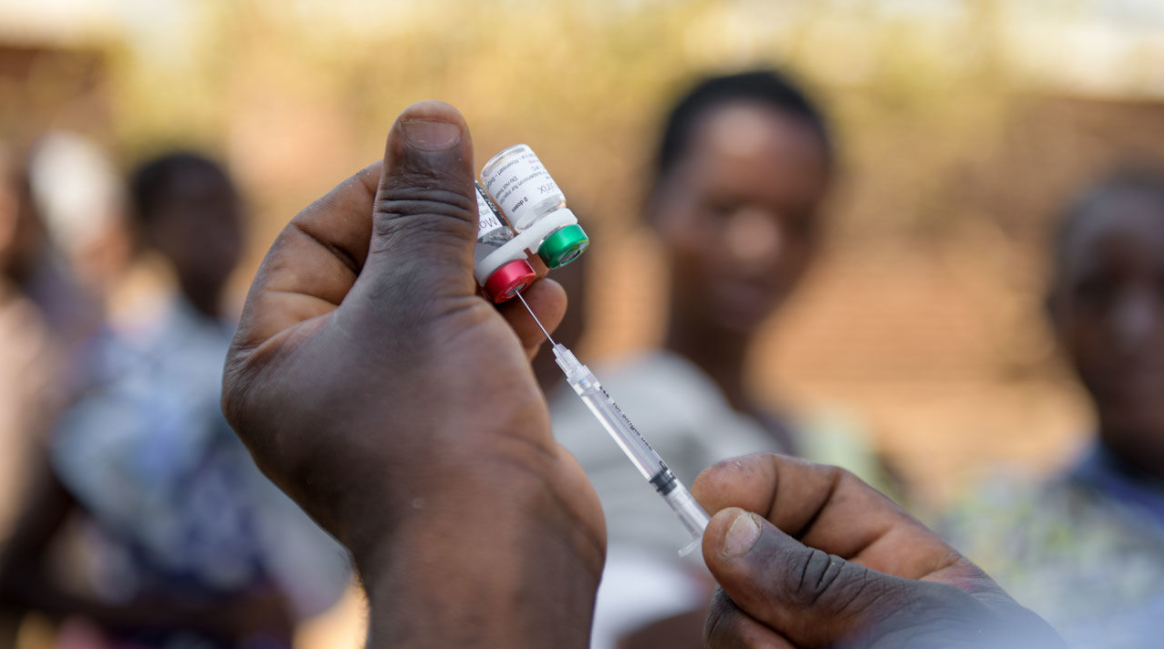
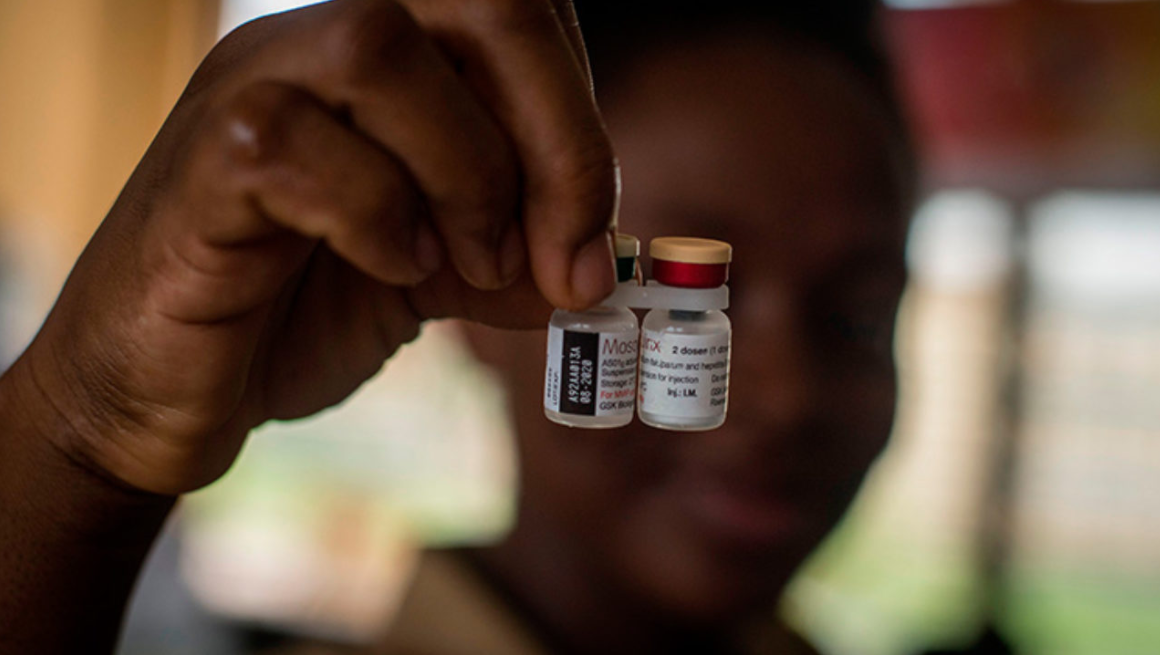
RTS,S/AS01 - The only vaccine so far that reach Phase III trial
More than a dozen vaccine candidates are now in clinical development, and one, GlaxoSmithKline Biologicals’ RTS,S, has completed Phase III clinical testing—the first malaria vaccine candidate to advance this far. RTS,S/AS01 (RTS,S) is a vaccine that acts against Plasmodium falciparum, the deadliest malaria parasite globally and the most prevalent in Africa.In January 2016, the vaccine was recommended by WHO for pilot introduction in selected areas of 3 African countries. RTS,S is being evaluated for use as a complementary malaria control tool that could be added to (and not replace) the core package of WHO-recommended preventive, diagnostic, and treatment measures.
Beginning in 2019, 3 sub-Saharan African countries – Ghana, Kenya, and Malawi – led the introduction of the vaccine in selected areas of moderate-to-high malaria transmission as part of a large-scale pilot program coordinated by WHO. The aim is to vaccinate about 360 000 children per year in the selected areas across the 3 countries. Vaccinations are being provided through each country’s routine immunization program, said WHO.
The Phase 3 trial, conducted over 5 years (from 2009 to 2014), enrolled approximately 15 000 young children and infants in 7 sub-Saharan African countries.* The trial sites within these countries represented a range of malaria transmission settings. Among children aged 5–17 months who received 4 doses of RTS,S, the vaccine prevented approximately 4 in 10 (39%) cases of malaria over 4 years of follow-up and about 3 in 10 (29%) cases of severe malaria, ** with significant reductions also seen in overall hospital admissions as well as in admissions due to malaria or severe anemia. The vaccine also reduced the need for blood transfusions, which are required to treat life-threatening malaria anemia by 29%.
RTS,S/AS01 side effects
Known side effects include pain and swelling at the injection site and fever. These side effects are similar to reactions observed with other vaccines given to children. The vaccine is associated with an increased risk of febrile seizures within 7 days of the administration. In the Phase 3 trial, children who had febrile seizures after vaccination recovered completely and there were no long-lasting consequences, said WHO.
However, NCBI pointed out that no significant efficacy against severe malaria was noted during the study period in young infants or children who did not receive a booster dose of vaccine. Vaccination, by providing protection against malaria infection, might have reduced the natural acquisition of immunity obtained through repeated infections, making these children more susceptible when the vaccine effect waned.
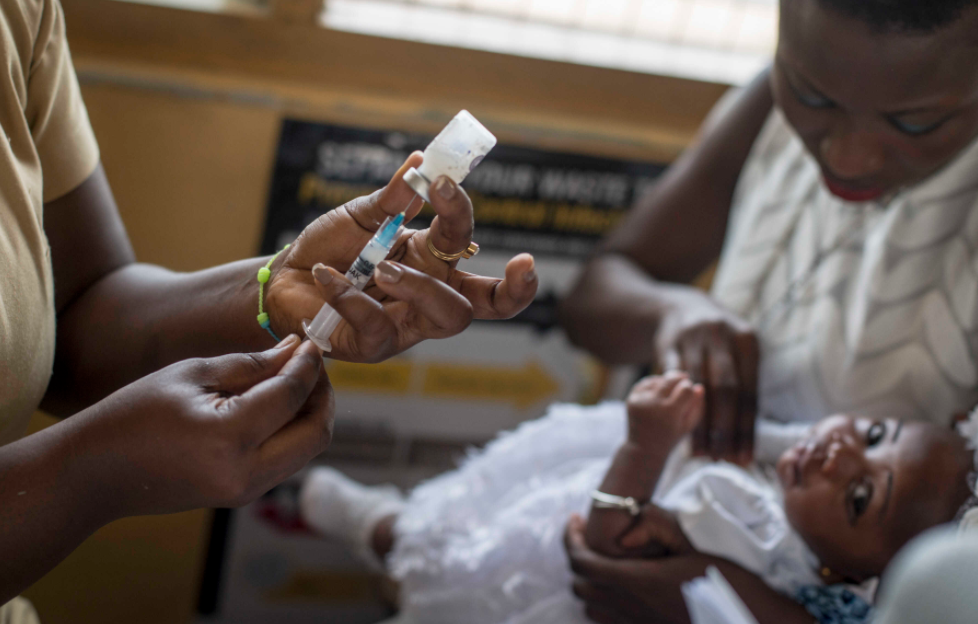 |
| Photo: AFP |
Malaria Vaccines: The Way Forward
According to CDC, other malaria vaccine candidates are in development or trial phases, including transmission-blocking vaccines that target the sexual stage of parasite development in the mosquito, The world’s leading global health organizations have developed the Malaria Vaccine Technology Roadmap for accelerating the development of a highly effective malaria vaccine.
The roadmap includes the following strategic goals for malaria vaccines by 2030 includes: (1) Develop and license malaria vaccines with protective efficacy of at least 75% against clinical malaria for areas with ongoing malaria transmission; and (2) Develop malaria vaccines that reduce transmission and human malaria infection, enabling elimination in multiple settings through mass vaccination campaigns.
 Recommendations for Pfizer Covid-19 vaccines injection Recommendations for Pfizer Covid-19 vaccines injection As almost vaccines carries warnings about the risk of allergic reactions and Pfizer Covid-19 vaccine is not excluded. |
 Facts about Chinese COVID-19 Vaccine? Facts about Chinese COVID-19 Vaccine? China also joined the race of Covid-19 vaccine production with some candidates undergoing final-stage clinical trials. Beijing boasts the effectiveness as well as the quantities ... |
 The COVID-19 Vaccine Global Race The COVID-19 Vaccine Global Race A race was called to fast find vaccines that could stop the spread of COVID-19. Now, nearly a year on from the start of the ... |