How to Convert Moles to Grams: Top Simple Steps
 |
| Illustrated picture. Photo: Mixi's |
Moles are a standard unit of measurement in chemistry that takes into account the different elements in a chemical compound. Often, amounts of compounds are given in grams and need to be converted to moles.
Grams and Moles of a Chemical Substance
Some of you might own a small scale in your kitchen, which you may have used to measure the amount of flour, sugar, and even meat. When you use your kitchen scale, you may get the amount of the substance in grams, ounces, or pounds. In the same way as in your kitchen, you need to measure the amount of substances in a chemistry laboratory. Chemists use a device called an analytical balance, which measures the amount of a chemical substance or compound, usually in grams.
Moles are the standard unit that is used in chemistry to express the amount of a chemical compound. Unfortunately, there is no method to measure moles directly, because we can only physically measure the amount of a compound in grams when we use an analytical balance.
The good thing is, it is possible for us to convert the number of grams of a compound to the number of moles.
Moles to Grams Conversion Problem
Sometimes you are given moles and need to convert it into grams. This worked example problem shows you how to convert moles to grams.
Problem
Determine the mass in grams of 3.60 mol of H2SO4.
Solution
First, look up the atomic masses for hydrogen, sulfur, and oxygen from the periodic table. The atomic mass is 1.008 for H, 32.06 for S, and 16.00 for O. The formula mass of H2SO4 is:
2(1.008) + 32.06 + 4(16.00) = 98.08
Thus, one mole of H2SO4 weighs 98.08 grams. This relation provides a conversion factor to go from grams to moles. Using the factor 98.08 g / 1 mol:
grams H2SO4 = 3.60 mol x 98.08 g / 1 mol = 353 g H2SO4
Answer
There are 353 grams of H2SO4 in 3.60 moles of H2SO4.
Moles to Grams Example
Question: What is the mass in grams of 4.80 moles of hydrogen peroxide (H2O2)?
Just like the first example, we need to know the molecular mass of hydrogen peroxide. H2O2 has two hydrogen atoms and two oxygen atoms.
atomic mass of H = 1.01 g/mol
atomic mass of O = 16.00 g/mol
molecular mass of H2O2 = 2⋅(atomic mass of H) + 2⋅(atomic mass of O)
molecular mass of H2O2 = 2⋅(1.01 g/mol) + 2⋅(16.00 g/mol)
molecular mass of H2O2 = 2.02 g/mol + 32.00 g/mol
molecular mass of H2O2 = 34.02 g/mol
Now we know 1 mole of H2O2 has a mass of 34.02 grams. This value gives us our conversion factor to find the mass of the H2O2.
mass of H2O2 = 4.80 mol x 34.2g/1mol = 163.30 grams
4.80 moles of hydrogen peroxide has a mass of 163.30 grams.
 How to Convert Grams to Moles How to Convert Grams to Moles The mole is a standard unit in chemistry and is a way to measure the amount of a substance. Check out the full detailed instructions ... |
Calculating the Molecular Mass
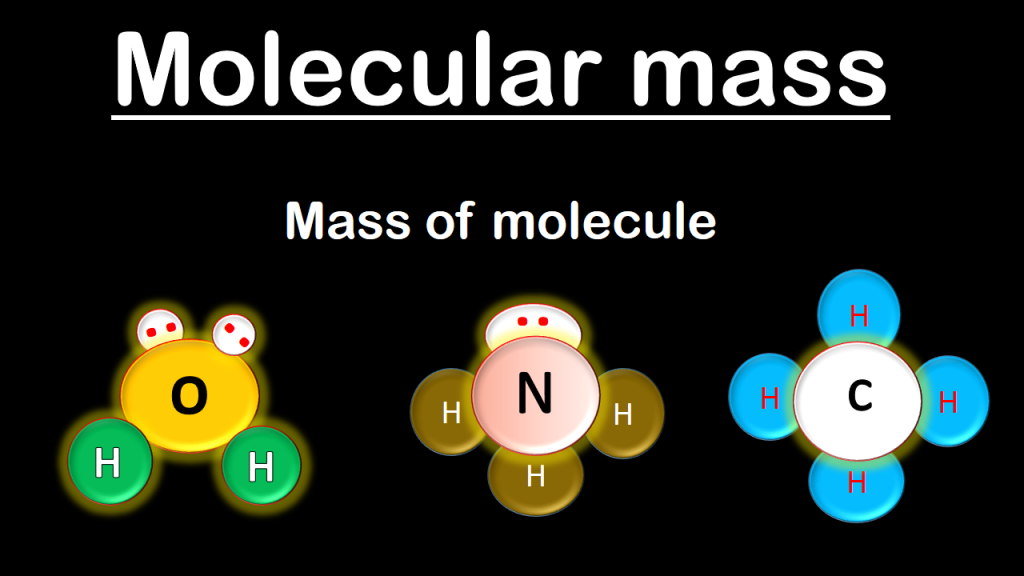 |
| Photo: Best Online Free Chemistry (Class 9 -12 ) |
Gather the necessary tools for solving a chemistry problem. Having everything you need easily accessible will simplify the process of solving the assigned problem. You will need the following:
- A pencil and paper. Calculations are easier to solve when you write them out. Be sure to show all your steps to get full credit.
- A periodic table. You will need to be able to find atomic weight of elements using the periodic table.
- A calculator. Calculators are necessary to simplify calculations of complex numbers.
Identify the elements in the compound that you need to convert into moles. The first step in calculating molecular mass is identifying each element that composes the compound. It is easy to distinguish elements because abbreviations contain only one or two letters.
- If a compound is abbreviated with two letters, the first will be capitalized while the second will be lowercase. For example, Mg is the abbreviation for magnesium.
- The compound NaHCO3 has four elements in it: sodium (Na), hydrogen (H), carbon (C), and oxygen (O).
Determine the number of atoms that each element contributes to the compound. You must know how many atoms of each element are present to calculate the molecular mass.The number of atoms each element contributes will be written in a subscript next to the element.
- For example, H2O has two atoms of hydrogen and one atom of oxygen.
- If a compound has parentheses followed by a subscript, each element within the parentheses gets multiplied by the number in the subscript. For example, (NH4)2S has two atoms of N, eight atoms of H, and one atom of S.
Write down the atomic weight of each element. A periodic table is the easiest way to find the atomic weight of an element. Once you locate the element on the table, the atomic weight is usually found underneath the symbol for that element.
- The atomic weight, or mass, or an element is given in atomic mass units (amu).
- For example, the molecular weight of oxygen is 15.99.
Calculate molecular mass. The molecular mass of a substance is calculated as the number of atoms of each element multiplied by the atomic weight of that element.Knowing the molecular mass is necessary to convert grams to moles.
- Multiply the number of atoms each element contributes to the compound by the atomic weight of that element.
- Add the total weight of each element in the compound together.
- For example, (NH4)2S has a molecular weight of (2 x 14.01) + (8 x 1.01) + (1 x 32.07) = 68.17 g/mol.
- Molecular mass is also referred to as molar mass.
| Key Takeaways: Converting Grams to Moles (and Vice Versa) * Grams and moles are two units to express the amount of matter in a sample. There is no "conversion formula" between the two units. Instead, you must use atomic mass values and the chemical formula to do the conversion. * To do this, look up atomic masses on the periodic table and use the formula mass to know how many atoms of each element are in a compound. * Remember, subscripts in a formula indicate number of atoms. If there is no subscript, it means there is only one atom of that element in the formula. * Multiply the number of atoms of an element by its atomic mass. Do this for all the atoms and add the values together to get the number of grams per mole. This is your conversion factor. |
In this video, you will know how to easily convert Moles to Grams:
For more interesting news of KnowInsiders, check out right below!
 How to Convert Minutes to Hours: Easy Ways to Change How to Convert Minutes to Hours: Easy Ways to Change How to convert minutes to hours? Follow our simple steps! |
 How to Convert PDF To Excel: Best Ways to Change How to Convert PDF To Excel: Best Ways to Change Your coworker sent you a PDF file instead of the Excel document you wanted, that doesn’t mean you’re actually stuck with it. In this guide, ... |
 How to Convert Word to PowerPoint How to Convert Word to PowerPoint PowerPoint is a great way to merge text and images for presentations. Are you struggling with the way to convert Word to Powerpoint? Check out ... |