India Covid-19 Update: Who will get First Dose of Vaccine and Domestic Vaccine?
 |
| Who will get First Dose of Covid-19 Vaccine in India? |
India Covid-19 update News - December 7
India reported over 36,000 new cases in 24 hours, taking the total tally over 96.4 lakh in the world’s second-worst infected country, according to the Health Ministry’s update as of 8 a.m on Dec. 6. That includes over 91 lakh recoveries and more than 1.4 lakh deaths.
Authorities reported nearly 42,000 recoveries in a day — the ninth straight day when new cases trailed recoveries.
Globally, cases exceeded 67 million with more than 1.53 million dead from the infection.
Key Figures Covid-19 in India:
Total number of confirmed coronavirus cases: 96,44,222
Active cases: 4,03,248
Cured/discharged/migrated: 91,00,732
Deaths: 1,40,182
Number of fresh cases in 24 hours: 36,011
One-day recoveries: 41,970
One-day deaths: 482
Update Covid-19 vaccine in India: Emergency use nod for AstraZeneca's COVID-19 vaccine
Serum Institute of India, the world’s largest vaccine producer by volume, has sought emergency use authorization in the country for AstraZeneca Plc’s COVID-19 vaccine on Sunday, according to several reports in Indian media.
The move comes close on the heels of Pfizer Inc applying for a similar authorization of its coronavirus vaccine in India on Saturday.
India purchase 1.6 billion doses of Covid-19 Vaccine
India is the largest buyer of COVID-19 vaccines in the world with 1.6 billion doses, that could cover 800 million people, or 60% of its population.
India has confirmed 1.6 billion doses of three vaccines as of November 30. India has purchased 500 million doses of the Oxford University-AstraZeneca vaccine candidate, 1 billion from the U.S. company Novavax and 100 million doses of the Sputnik V candidate from Russia’s Gamaleya Research Institute, according to the U.S.-based Duke University Global Health Innovation Center.
Countries with manufacturing capacity, such as India and Brazil, have been successful in negotiating large advance market commitments with leading vaccine candidates as part of the manufacturing agreements, the Duke researchers noted in their analysis.
Who will get First Dose of Covid-19 Vaccine in India?
Bharat Biotech and Zydus-Cadila would also add about 400 million doses annually. In brief, the numbers appear reasonable over 2021 and 2022, said Dr. Jameel, director of the Trivedi School of Biosciences, Ashoka University.
“We can expect the first 250 million to be vaccinated in 2021; the remaining in the following years. The problem will not be vaccine doses, but the ability to deliver them,”Dr. Jameel explained.
The first 500 million doses, the virologist said, are likely to go to 250 million people in the group that includes frontline workers, healthcare workers, sanitation, emergency services, and security services. This group also includes the elderly above 65 and patients with comorbidities.
However, immunologist Satyajit Rath said there is currently no particularly reliable estimate of the percentage of community coverage needed to be vaccinated in order to reach the point of ‘herd immunity’. The reliability of models being used for these estimations is far from clear, he stressed to The Hindu.
Pfizer India to seek emergency-use authorisation of Covid-19 vaccine in India
Pfizer India has become the first pharmaceutical firm to seek from the Drugs Controller General of India (DCGI) an emergency-use authorisation for its Covid-19 vaccine in the country, after its parent company secured such clearance in the UK and Bahrain.
"The firm has submitted the EUA application in Form CT-18 for grant of permission to import and market Pfizer-BioNTech's Covid-19 mRNA vaccine BNT162b2 in the country," the source of Business-standard said.
However, top officials monitoring India’s vaccine plan said that Pfizer’s vaccine may not meet the country’s "immediate" domestic requirements given that the firm has prior delivery commitments with several countries.
*Discover full Story concerning Pfizer Covid-19 vaccine, please CLICK HERE
Coronavirus Latest News in India
Today, November 6, Total COVID-19 cases in India have reached 96.08 lakh. There are 409,689 active cases in the country. India implemented a nationwide lockdown to help curb the novel coronavirus pandemic.
So far, India has recorded 9,608,211 confirmed COVID- 19 cases, including 139,700 deaths. A total of 9,058,822 people have recuperated from COVID-19 till 8 am on December 5, as per data from the Ministry of Health & Family Welfare (MoH).
 |
| So far, India has recorded 9,608,211 confirmed COVID- 19 cases, including 139,700 deaths |
*The tally of COVID-19 cases in Punjab rose to 1,55,424 on December 5 with 644 new cases of the infection, while 23 more fatalities pushed the death toll to 4,905.
*Uttar Pradesh reported 1,940 new COVID-19 cases on Saturday, taking the infection tally to 5,53,012, while 23 more fatalities pushed the state's death toll to 7,900.
*Tamil Nadu on December 5 reported 1,366 new COVID-19 cases, taking the overall infection count to 7,88,920, while 15 more fatalities pushed the death toll to 11,777.
*Gujarat on December 5 reported 1,514 fresh coronavirus positive cases, taking the total count of infections to 2,17,333, the state health department said.
*Maharashtra on December 5 reported 4,922 fresh COVID-19 cases, taking the count of infections to 18,47,509, the state health department said.
*Kerala reported 5,848 fresh COVID-19 cases on December 5, taking the total caseload to over 6.31 lakh, while the toll mounted to 2,390 with32 more fatalities. While 5,820 people have been cured, the total recoveries so far has touched 5,67,694.
India’s domestic Covid-19 Vaccine
India’s domestic Covid-19 vaccine candidate by Bharat Biotech also entered Phase 3 clinical trials this week.
Haryana health minister Anil Vij, who was administered the trial dose of the indigenous Covid-19 vaccine Covaxin last month, has tested positive for coronavirus on Saturday. Bharat Biotech, the developer of the vaccine, clarified that its efficacy can only be determined 14 days after the second dose.
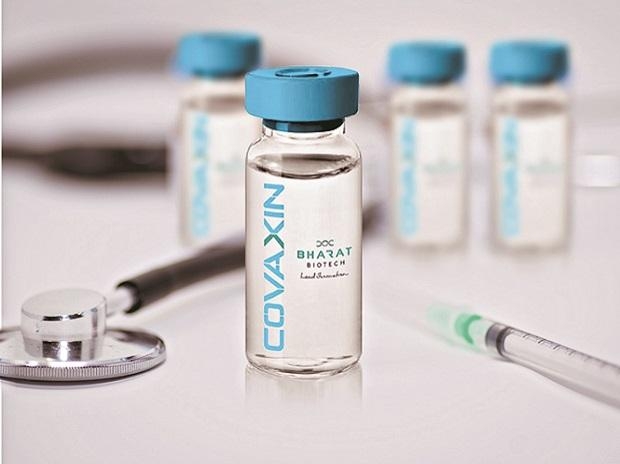 |
| the Covaxin clinical trials are based on a 2-dose routine, the two doses are administered 28 days apart and vaccine efficacy will be determined 14 days post the second dose |
States that have started phase 3 trials of Covaxin in India: The Covaxin phase 3 trials have commenced in several states in the country, including Haryana, West Bengal, Madhya Pradesh and other states are also expected to begin soon. It is the largest clinical trial conducted for a coronavirus vaccine in India
The trials of its Covid-19 vaccine candidate -- Covaxin -- which involves 26,000 participants across 22 sites in the country, began on 16 November. It is the largest clinical trial conducted for a coronavirus vaccine in India.
The Covaxin phase 3 trials have commenced in several states in the country, including Delhi, Karnataka and Bengal, and many other states are expected to begin soon.
| Uttar Pradesh has become the first state to have tested more than two crore samples for COVID-19, said Additional Chief Secretary Medical and Health Amit Mohan Prasad on December 5. "No state has conducted so many tests so far. Yesterday 1,66,938 samples were tested whereas a total of 21,028,312 samples have been tested so far across the state," said Prasad. |
Besides the Hyderabad-based firm's Covaxin in the country, four other potential vaccines are under various phases of human clinical trial with the Serum Institute of India conducting phase-three trial of the Oxford-Astrazeneca Covid-19 vaccine while the indigenously developed vaccine by Zydus Cadila has completed phase-two clinical trial.
India the focus remains on five other candidates, including one developed by AstraZeneca and Oxford University and produced by the Pune-based Serum Institute.
Last week AstraZeneca said its candidate - Covishield - could be around 90 per cent effective on following one of two dosing regimens. The average efficacy of two regimens was 70 per cent.
Covishield, which is at the head of the list of vaccine candidates likely to be rolled out in India, will be produced by the Serum Institute, which has committed to making at least 100 million doses available by the end of January and hundreds of million by the end of February.
*Latest updates on Covid-19 vaccines around the World
States in India that have started the phase 3 trials of Covid-19 Domestic Vaccine1) Haryana - Haryana became the first state to start Covaxin phase 3 trials on 20 November with state home minister Anil Vij being the first volunteer to be administered a trial shot. Vij was administered the trial dose at the Civil Hospital at Ambala Cantt. 2) Delhi- The phase 3 human clinical trial of Covaxin began at the AIIMS in the national capital on 26 November. The trial began as Dr M V Padma Srivastava, the chief of Neurosciences Centre at the premier institute, and three other volunteers received the first dose. The trail doses were reportedly given to around 15,000 volunteers in Delhi's AIIMS. 3) West Bengal: Governor Jagdeep Dhankhar today inaugurated the launch of Phase-3 regulatory trial of Covaxin at Kolkata's ICMR-National Institute of Cholera and Enteric Diseases (ICMR-NICED), lauding India's leadership for "effectively tackling" the novel coronavirus pandemic. Bengal minister Firhad Hakim became the first volunteer to take the Covid-19 vaccine 'Covaxin'. 4) Gujarat: Phase 3 trials of Covaxin commenced in Gujarat on 26 November at Ahmedabad’s Sola Civil Hospital, the only site in the state where the exercise will be carried out. The Sola civil hospital is one among the 130 centres across the country where the third phase of clinical trials of Covaxin would be conducted. 5) Odisha - The third phase human trial of Covaxin, the much-awaited indigenously developed Covid-19 vaccine, began at an institute in the state on 20 November. The vaccine was administered to two recruits at the Preventive and Therapeutic Clinical Trial Unit (PTCTU) at the Institute of Medical Sciences and SUM Hospital, the only institute in Odisha chosen by the ICMR for the human trial of the vaccine. 6) Madhya Pradesh - People’s University in Bhopal is the site for phase 3 trials of Covaxin in Madhya Pradesh. The trial vaccination had started on 27 November. |
What is COVAXIN - India's First Domestic COVID-19 Vaccine
COVAXIN, India's indigenous COVID-19 vaccine by Bharat Biotech is developed in collaboration with the Indian Council of Medical Research (ICMR) - National Institute of Virology (NIV). The indigenous, inactivated vaccine is developed and manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) high containment facility.
The vaccine received DCGI approval for Phase I & II Human Clinical Trials and the trials commenced across India from July, 2020.
 |
| Haryana Health Minister was earlier administered the trial dose of Covaxin vaccine. (File photo: PTI) |
After successful completion of the interim analysis from the Phase 1 & 2 clinical trials of COVAXINTM, Bharat Biotech received DCGI approval for Phase 3 clinical trials in 26,000 participants in over 25 centres across India.
Haryana Health Minister Anil Vij has tested positive for coronavirus days after participating in the trial of coronavirus vaccine Covaxin. On November 20, Vij had participated in a trial of coronavirus vaccine Covaxin.

























