Can Dostarlimab Medicine Cure All Types of Cancer?
 |
| Dostarlimab drug to treat cancer |
| Table of Content |
The whole world was excited by the news of the first drug to completely cure rectal cancer. The drug, Dostarlimab, was announced after a small trial in the US in which 12 patients with rectal cancer were in 100% remission after using the drug for about 6 months.
Dostarlimab is considered a historic breakthrough in the treatment of 100% of cancers.
Doctors and scientists are hopeful that the research further shall provide positive results and the therapy is availed to the 18.1 million cancer patients around the world.
However, can Dostarlimab drug cure all types of cancer?
According to Dr. Aju Mathew, an expert at Ernakulam Cancer Center, inhibitors such as dostarlimab are only suitable for patients with impaired function of the DNA pairing repair (MMR) system.
These patients have certain genetic mutations that cause the repair system to fail to match the DNA in the cell. Uncorrected cells often have many genetic mutations, which can lead to cancer.
However, this condition occurs in less than 10% of colon cancer patients. Therefore, the drug is unlikely to be effective in these patients.
MMR is most common in people with colorectal cancer, gastrointestinal cancers, and endometrial cancer. Some breast, thyroid, prostate, and bladder cancer patients also experience this condition.
Is Dostarlimab medicine a "panacea" for cancer?
Over the past few days, Dr. Mathew has received countless questions about whether this is a miracle, completely cured cancer. He acknowledges dostarlimab as a promising drug, but notes that clinical trials have been very small, with results only on patients with a specific type of cancer.
Nitesh Rohatgi, senior director, department of oncology, Fortis Memorial Research Institute, said that dostarlimab should be prescribed with certain other drugs, rather than alone.
According to Dr CS Pramesh, director of the Tata Memorial Center in Mumbai, calling dostarlimab "a cancer panacea that could impact global treatment" is premature and far-fetched.
"While the study results are interesting, we need to treat, monitor a large number of patients, do a larger randomized trial before we can conclude this drug is a game changer," he said.
According to Dr. Rohatgi, with the new study results, the community should at this point expect only one colon cancer immunotherapy, which can be used before surgery.
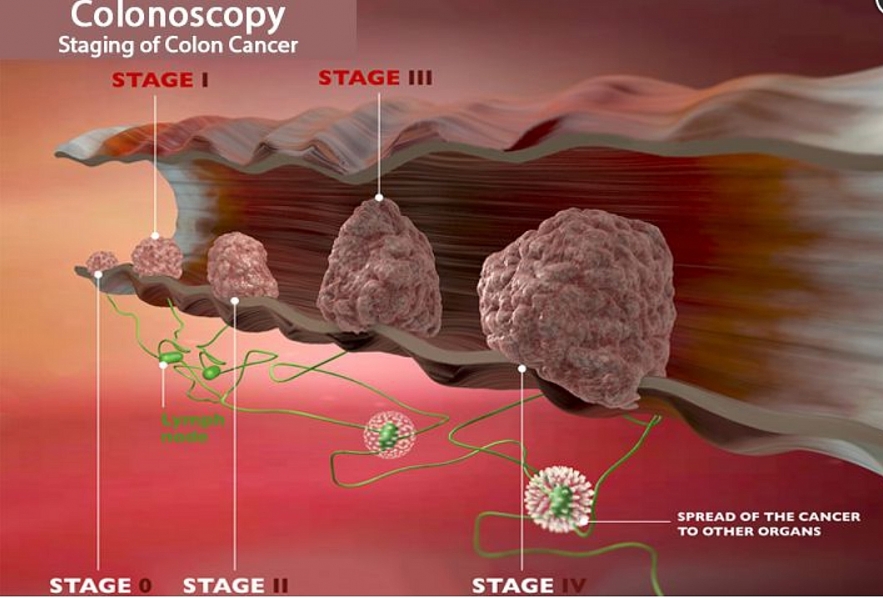 |
| The limitation of this study is that it was only performed on 12 patients with stage II and III colorectal cancer who carried the MMRd mutation in the tumor. This is only a small group representing about 5% of patients with colorectal cancer. |
Next, scientists need to perform genomic analysis, understand the biology of cancer and find specific treatments for specific patients, said Dr. Sewanti Limaye, director of the oncology department at the Sir HN Reliance Foundation hospital, said.
In an accompanying editorial, University of North Carolina gastrointestinal oncologist Hanna Sanoff said the findings were "cause for great optimism", but the drug would not be able to replace current treatments just yet.
This study only focused on a very small sample size, namely 12 people. It could highlight the randomness, when all 12 people responded well to the drug treatment.
In addition, all of these patients were carefully selected. They all belong to a subgroup of only 5% of colorectal cancer patients who have MMRd mutations in their tumors.
This is a mutation that impairs their ability to repair DNA and makes them particularly susceptible to control factor inhibitors such as dostarlimab.
For the 95% of colorectal cancer patients who do not have this mutation, we cannot currently assess the effects of dostarlimab.
Certainly in the future, doctors at Memorial Sloan Kettering Cancer Center will have to expand their trial to phase III, where they must design larger, more randomized, and controlled participants groups double blind form. Only then will we have the final answer to the effectiveness of dostarlimab drug.
Until then, the treatment regimen for colorectal cancer patients, especially those without MMRd mutations, will remain unchanged. It is perfectly reasonable for doctors to continue to give their patients standard treatment, which includes chemotherapy and radiation to shrink the tumor followed by surgery.
We must understand that 100% successful treatment results (for the number of 12/12 patients) belonging to the group of 5% of colorectal cancer patients with MMRd mutations are not absolute numbers.
Dostarlimab medicine costs
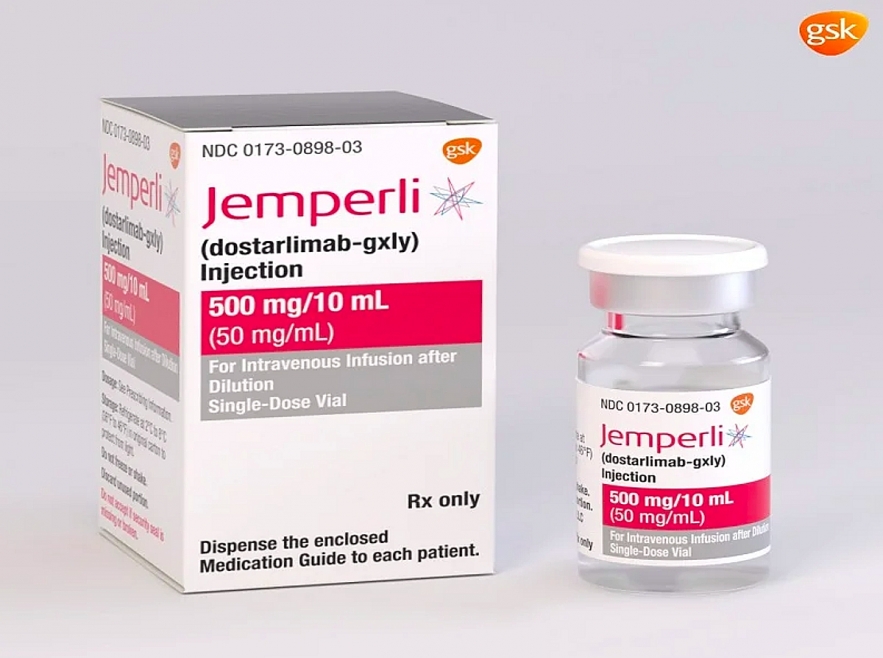
The drug they used, dostarlimab, was developed by GlaxoSmithKline. It's what's known as a "checkpoint inhibitor" and is a current treatment for mismatch-repair-deficient endometrial cancer.
It's not cheap — each 500mg dose costs $15,500 in the US. In the UK, it is sold for £5,887 per dose. However, the NHS has agreed a discount with the manufacturer GlaxoSmithKline (GSK), which sponsored the US trial, to treat advanced endometrial cancer.
But it works by "unmasking" tumours. Cancer cells have a bunch of tricks that let them grow and spread while flying under our immune system's radar.
The researchers acknowledge their study is just the beginning, and envision dostarlimab will be trialled in other localised tumours with DNA mismatch deficiency, such as some pancreatic, stomach and prostate cancers.
 What is Dostarlimab: Drug of 100% Cure for Cancer What is Dostarlimab: Drug of 100% Cure for Cancer For the first time in history, a small clinical trial in the United States showed 100% eradication of the cancer disease in patients by Dostarlimab. ... |























